Search and Discovery
Study tracks the changes in a vision protein as fish evolved
Genetic engineering, statistics, and spectroscopy are combined to address a fundamental question in biology.
How does Darwinian evolution work on the molecular scale? That is, how do genetic mutations, which occur in single molecules of DNA, lead to changes that help an organism of 1023 or so molecules adapt to its environment?
The answer might seem straightforward. Because DNA encodes proteins, evolution presumably entails proteins becoming better at their jobs and, through improved performance, bestowing an advantage on a plant or animal as it struggles to survive.
Thanks to gene sequences and statistical algorithms, one can often infer the mutations a protein has undergone through the ages. And thanks to genetic engineering, one can coax certain cells into making a protein of one's choice, even a protein from a long-extinct species.
Measuring a protein's evolving job performance is harder. Proteins fulfill their roles by binding to other molecules. In most cases the consequences of that binding are hard to observe. But, as Emory University's Shozo Yokoyama realized, there is at least one class of protein, opsins, whose function has a clear, quantifiable signature.
Opsins help fish and other animals see in dim light. The molecule ultimately responsible for vision is retinal, a light-sensitive derivative of vitamin A. Isolated retinal absorbs in the UV, but when it's swaddled by a much larger opsin, retinal's peak absorption wavelength, λmax, shifts redward.
Depending on its amino acid sequence, an opsin can shift λmax from the UV all the way through the colors of the rainbow to the IR. Fish exploit opsin tuning ability to evade predators during twilight, a particularly dangerous time of day.
In shallow, clear water, the twilight spectrum peaks broadly between 400 nm (violet) and 500 nm (green). In deep water, the spectrum narrows around a peak at 480 nm (blue). And in shallow, muddy water, the spectrum shifts redward.
As you might expect, fish that swim today in those diverse habitats have opsins that engender an appropriate, survival-enhancing shift. Do those shifts constitute proof that Darwinian evolution works on the molecular scale? Not quite.
What's missing is evidence that opsins have repeatedly changed over the ages as fish spread into new environmental niches and diversified. To look for that evidence, Yokoyama and his collaborators undertook a seven-year project. They inferred the gene sequences of a family tree of fish opsins, re-created those opsins, and measured the λmax values in the lab.1
The results, which have just been published, provide strong circumstantial evidence of molecular evolution. They also reveal the limitations of using statistical techniques alone to identify adaptive mutations in proteins.
Family ties
Yokoyama chose to focus on eight species: two eels that migrate from deep water to shallow water (Japanese eel, Japanese conger), five deep-sea fish (Pacific blackdragon, Northern lampfish, shining loosejaw, scabbardfish, viperfish), and one fish that lives in shallow freshwater (bluefin killifish).
The eight fish have opsins that suit their diverse habitats. Indeed, the Japanese eel has two opsins: one optimized for the shallow water where it lives, another for deep water where it spawns. The shining loosejaw glows luminescently in the IR. Its opsin is tuned to the IR, presumably to see the prey it illuminates, find its fellow shining loosejaws, or both.
To trace how those opsins might have evolved, Yokoyama first applied a statistical technique called phylogenetic reconstruction. As input he used the genetic sequences of 38 opsins drawn from the eight species, supplemented by those of other fish and vertebrates.
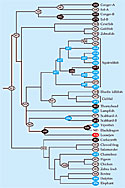
The result is the family tree of opsins in figure 1. Its trunk springs from the ancestor of today's vertebrates, an animal that likely swam in fresh, shallow water more than 400 million years ago.
Each node of the tree, downstream of the trunk and upstream of the present-day vertebrates, represents the opsin of an extinct species. Applying the techniques of genetic engineering, the Emory team re-created those ancestral opsins in the lab and equipped them with retinal molecules. Then they measured λmax.
The combination of spectral measurements and genetic sequences revealed which DNA mutations—that is, amino acid substitutions—were responsible for the shift in λmax.
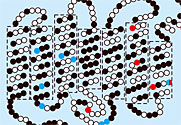
Vertebrate opsins, extinct and extant, differ slightly in the total number of amino acids. Those differences in length appear at the protein's two tails. Mutations aside, the protein's functional core has remained more or less constant. It consists of seven helices that form a tube to hold the retinal.
A flattened representation of bovine opsin appears in figure 2. Black, red, and blue dots indicate amino acid sites where the Emory team found substitutions in its opsin family tree. Substitutions at blue sites decreased λmax; substitutions at red sites increased λmax; substitutions at black sites left λmax unchanged.
Bovine opsin has 354 amino acids. Substitutions at just 12 sites, 3%, could account for all the variation in λmax that appeared in the opsin family tree.
Which substitutions changed λmax proved surprising. In previous work, Yokoyama had found that substituting alanine with serine at the 292nd site (A292S) usually decreases λmax by 10 nm. That substitution was acquired by one of the conger eel opsins from its immediate ancestor. Making the reverse substitution (S292A) in the conger eel opsin increased λmax by 10 nm, as you'd expect.
Here's the surprise. When A292S was applied to the immediate ancestor, it had no effect on λmax! Evidently, two other substitutions found by the Emory team were responsible for the decrease, not A292S. Other cases occurred in which quite different substitutions could shift λmax by more or less the same amount.
Opsins are important not only for vision; they are also candidates for light-switched, high-density data storage. When physicists and chemists calculate λmax, they typically focus on amino acids that lie within 4.5 Å of retinal. However, the substitutions that blueshifted the conger opsin's λmax occurred at sites 20 Å away from retinal. Moreover, as figure 2 shows, some sites that shifted λmax don't even belong to opsin's functional core.
A bigger surprise occurred when Yokoyama ran the opsins' genetic sequences through programs designed to identify naturally selected mutations. Three DNA bases suffice to encode a particular amino acid, but the coding has redundancies. For example, GCT, GCC, GCA, and GCG all encode alanine. Changing the third base in any of those four codons would still yield alanine.
Changing the first base from guanine (G) to cytosine (C) would yield a different amino acid, proline. Usually, such "nonsynonymous" substitutions impair a protein's ability to fold or function. They aren't passed on to the next generation.
But some nonsynonymous substitutions turn out to be beneficial. As DNA mutates randomly from generation to generation and from species to species, more synonymous substitutions than nonsynonymous substitutions accumulate. Any nonsynonymous substitutions that get passed on are likely to be evolutionarily significant.
Statistical algorithms implement those expectations. They digest a family tree of genetic sequences and, by comparing rates of synonymous and nonsynonymous mutations, identify putative mutations that constitute evolutionary adaptations.
Yokoyama had determined which mutations really do change λmax. Could the statistical methods find them too? The answer turned out to be no. Why that should be the case isn't clear. Ziheng Yang of University College London has developed some of the statistical methods used by evolutionary biologists. Those methods, he points out, may lack the sensitivity to detect adaptive mutations that become fixed within a short period of time, as may be the case in vertebrate opsins.
Gavin Naylor of Florida State University in Tallahassee points out another possible limitation: Statistical methods don't take into account the many-body nature of proteins. "Shozo shows quite compellingly that just because you change one site doesn't mean you're going to get a functional change," he says. "It depends, rather, on the context of the other amino acids."
Showing conclusively how the survival of the fittest plays out on the molecular level would require reconstructing not only the protein but also the whole organism. And you'd have to observe the organism and its descendants in their original, long-lost habitats.
Still, the Emory researchers did find a piece of reassuring evidence: The opsin of the vertebrates' piscine ancestor tuned retinal to absorb at 501 nm. That λmax is consistent with the shallow-water habitat of its fossilized remains.
Charles Day
Reference
-
1. S. Yokoyama, T. Tada, H. Zhang, L. Britt, Proc. Natl. Acad. Sci. USA 105, 13480 (2008) [MEDLINE].
-
Free this month
-
Study tracks the changes in a vision protein as fish evolved
-
Most popular articles
-
Home photovoltaic systems for physicists
July 2008 -
Ehrenfest letters surface
June 2008 -
Dispelling myths and highlighting history of the heliocentric model
June 2008